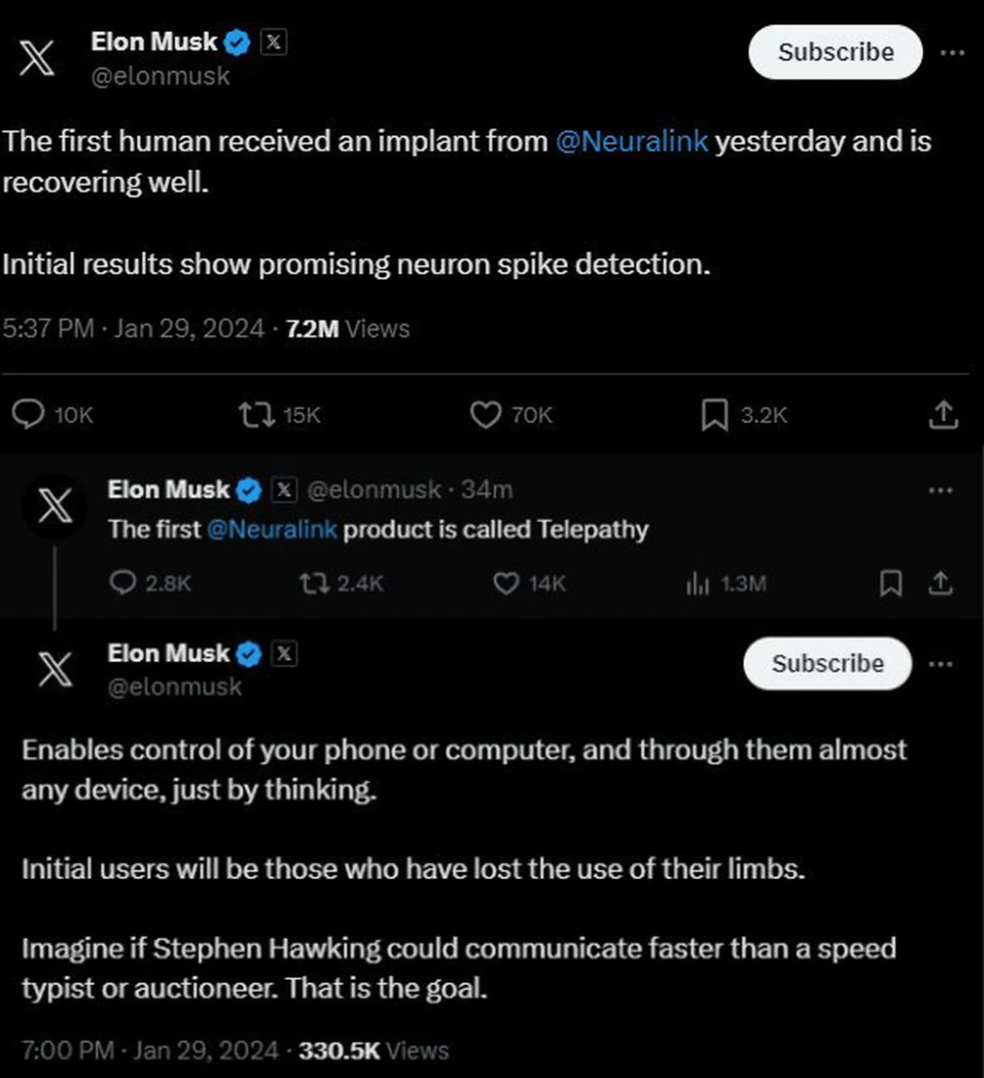
Elon Musk, the billionaire entrepreneur, investor, and technologist, recently announced that Neuralink had successfully implanted its brain-computer interface into a human for the first time. Musk posted on X (formerly Twitter) that the recipient was “recovering well” and that the “initial results show promising neuron spike detection.”
The ongoing trial by Neuralink, referred to as The PRIME Study, focuses on helping individuals with quadriplegia resulting from vertical spinal cord injury or ALS to control external devices using their thoughts. Experts believe the brain-computer interface (BCI) technology employed in this study holds significant potential applications, especially for individuals with disabilities.
Following FDA approval last May, Neuralink began recruiting human test subjects for the trial, focused on paralysis control technology. Scheduled to span six years, it will evaluate the effectiveness and safety of the N1 implant, the company's brain-computer device, the surgical process conducted by the R1 robot, and the usage of the N1 User App. As reported by The Verge, the trial targets individuals with quadriplegia over the age of 22, with a "consistent and reliable caregiver."
This technology holds immense promise for improving the lives of those grappling with paralysis. With an estimated 5.4 million individuals facing paralysis in the United States alone, the potential impact on the quality of life for millions is significant. However, it is important to recognize that Musk's intentions extend beyond aiding paralyzed individuals. Musk has not been shy about sharing his broader, long-term ambition “to achieve a symbiosis with artificial intelligence.” He has openly shared his interest in developing this technology to help humans to “merg[e] with AI” to prevent us from, in his words, being “left behind.”
This either visionary or fantastical goal, depending on how one sees it, raises eyebrows in the regulatory landscape. The FDA typically does not approve such human trials. Yet, because the current focus is on helping individuals with paralysis, they have been receptive to the technology.
Regardless of FDA approval, allegations from former Neuralink employees and field experts suggest that the company may be pursuing an unnecessarily invasive and potentially perilous approach to implants. The implants, designed to interface with the human brain, have been widely reported for causing damage in animal test subjects. These allegations hint at a potential prioritization of Musk's goal of merging with AI over the safety and well-being of the individuals involved.
The development of brain-connecting technologies has prompted a discussion about the broader ethical considerations beyond immediate concerns about Neuralink, which is not alone in its pursuits. Technologies that facilitate an interface with the human brain will enable a process for decoding mental processes, which will pose significant ethical risks. Mental privacy may soon be a legitimate concern, with the ability to decode and encode the inner workings of the brain. As brain-connecting technologies become safe, functional, and reliable, the vast array of implications for merging humans with AI will be a societal imperative.